 Position Documents
Position DocumentsControl of indoor environments for the well-being of occupants is one of the ASHRAE’s founding principles:
"The Society is organized and operated for the exclusive purpose of advancing the arts and sciences of heating, refrigeration, air conditioning, and ventilation, and the allied arts and sciences and related human factors for the benefit of the general public, as defined in the Certificate of Consolidation. To fulfill its role, the Society shall recognize the effect of its technology on the environment and natural resources to protect the welfare of posterity." (ASHRAE Bylaws, Article I, Section 1.3)
Consistent with this responsibility, the following is the Society’s position on legionellosis.
A presidential ad hoc committee was appointed in September 1980 to evaluate Legionnaires’ disease. Members of ASHRAE as well as consultants from industry, private practice, government, and academic institutions, having scientific, medical, or engineering expertise on legionellosis, were appointed to the committee.
The work of this ad hoc committee resulted in: (1) a Position Statement that delineated the nature of the problem and discussed possible approaches for its solutions, and (2) a two-part Position Paper that presented basic information known at that time about the disease and its causative agents; a discussion of environmental, energy, and economic implications of the disease; and recommendations for future action. This paper is the second update and condensation of these previous documents.
Legionellosis is an illness that has two distinct clinical forms. The most serious form is a pneumonia illness that is commonly referred to as Legionnaires’ disease. Generally, in epidemic settings, the proportion of people who contract the disease is 5% or less; however, risk factors such as age, smoking, underlying disease, and compromised immunity play a major role in susceptibility. The incubation period is 2 to 10 days, and treatment with erythromycin with or without rifampin is usually effective. Fatality rates in outbreaks have averaged about 15%. Early diagnosis and treatment (possibly including hospitalization) will reduce the chance of fatality, although the chance of succumbing to the disease may be higher among particularly vulnerable people.
The other clinical illness, Pontiac fever, is a flu-like illness without pneumonia. In contrast to Legionnaires’ disease, most of those who are exposed develop illness within 48 to 72 hours and most recover without antibiotic therapy.,
Legionellosis is caused by Legionella, a type of bacteria discovered in 1977. These microbes measure about 0.3 µm by 2 µm. When microorganisms grow their numbers increase. Individual cells repeatedly divide with new cells dividing again and again. The result can be millions of cells after a few hours or days. Legionella do not grow on ordinary laboratory media, but grow best when supplemented with L-cysteine, soluble iron and near a pH of 6.9. In spite of the strict nutritional requirements in the laboratory, these organisms are found in a wide variety of fresh waters,, and are found commonly in lakes and streams. Several investigators have reported nutritional relationships between legionellae and other bacteria, algae, and protozoa.,
For epidemiologic purposes, further characterization of organisms, such as Legionella pneumophila serogroup 1, can be done using monoclonal antibodies, multilocus enzyme assay (MEA), ribotyping, restriction fragment length polymorphism (RFLP), random amplified polymorphic DNA (RAPD), and pulsed-field-gel electrophoresis (PFGE) procedures. These techniques are commonly called "finger printing", and can be used to correlate the specific Legionella strain from an infected patient with an environmental source.
Legionella pneumophila can grow in tap water at 25° C to 42° C (77° F to 108° F) with an optimal temperature for growth of 37° C (98.6° F). Growth can also occur outside this range but the environment becomes more hostile the further from the optimal.
Currently, there are more than 35 species with numerous serogroups within some species. To date, roughly one-half have been associated with disease.
Legionnaires’ disease quickly gained public attention with the outbreak that occurred in the summer of 1976 among conventioneers attending the meeting of the Pennsylvania Division of the American Legion in Philadelphia. Because of the size and severity of the outbreak, federal, state, and local health authorities launched the largest cooperative investigation in history (before 1980) to determine the cause of the outbreak.
People who developed illness did so between 2 and 10 days after they had attended meetings in the convention center’s hotel. High fever, chills, headaches, and muscle aches and pains were the initial symptoms. Some people had dry coughs, chest pains, shortness of breath, mental confusion, vomiting, and diarrhea. All eventually developed pneumonia. Ultimately, 34 of the 221 people who became ill died. No person-to-person spread was documented in this outbreak or any others. Careful epidemiologic investigation eliminated a multitude of possible sources. Air was implicated as the probable pathway of infection. It was shown statistically that patients had spent a significantly longer period of time in the lobby of the convention center’s hotel than those who remained healthy.
Examination of sera and tissue specimens from some previously unsolved outbreaks that had been stored at the Centers for Disease Control (CDC) in Atlanta, Georgia, and elsewhere revealed that outbreaks of this disease had occurred earlier. The earliest case so far documented occurred in a soldier who had an unidentified pneumonia in 1947 when he was at Fort Bragg, North Carolina. The earliest outbreak (three or more cases from the same source) of legionellosis appears to have occurred in a meat packing plant in 1957.
Since the Philadelphia outbreak, additional outbreaks and numerous sporadic cases of legionellosis have been reported. About 1,400 cases are officially reported annually to the CDC, and about 500 are confirmed. A study of people hospitalized with pneumonia calculated that 11,000 cases of legionellosis occur annually in the United States. This calculation is thought to be an underestimate. Estimates as high as 100,000 cases annually in the U.S. have been suggested, although the scientific basis for these estimates is not clear. Serologic surveys indicate that many people in the general population have antibodies to legionellae in their blood. This suggests that large numbers of people may have been exposed and possibly infected with legionellae., While most of the earlier outbreaks were traced to aerosols contaminated with these organisms from either cooling towers or evaporative condensers,,,,, other outbreaks have been traced to potable water services and components, such as water heaters, showers, faucets, whirlpool baths,,,,, respiratory therapy equipment, decorative fountains,, and an ultrasonic mister sometimes used in grocery stores.
The first documented outbreak of Pontiac fever occurred in 1968 in Pontiac, Michigan. Ninety-five percent of the employees at the Oakland County Health Department developed illness, which generally lasted 2 to 3 days and consisted of fever, headache, and generalized muscle aching. All of the 144 people who became ill recovered. Ultimately, Legionella pneumophila serogroup 1 bacteria were isolated from condensate water collected from the drip pan of an evaporative condenser located in the building’s basement. In subsequent outbreaks of Pontiac fever Legionella pneumophila, L. anisa, L. feeleii3, and L. mcdadeii were implicated as the causative agents.
4.0 Transmission Factors and Prevention

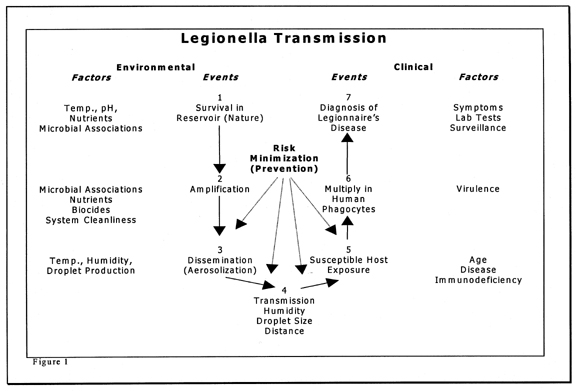
Prior to developing actual disease, a number of events must occur. Some of these events can be influenced by good engineering and maintenance practices. These events and prevention opportunities are outlined in Figure 1. The first event is generally outside the scope of building engineering and management practices. Events 2 to 4 can be influenced by engineering design and maintenance procedures. Later events are influenced by individuals’ health care practices.
The most effective control for most diseases including legionellosis is preventing transmission at as many points as possible in the diseases’ chain of transmission. The rationale for this is that if one preventative measure fails, others will be present and act as fail-safe mechanisms. With this philosophy in mind, each step for possible transmission of Legionella are presented and possible intervention strategies discussed. General concepts are presented so that readers may develop an understanding of the types of conditions that may allow amplification and transmission of Legionella.
Water is the reservoir for legionellae in the environment. Many lakes and streams have been found to harbor legionellae., Theoretically, the ultimate method for preventing human infections of legionellosis would be to completely eliminate or eradicate legionellae from the environment; however, this is impossible because of the ubiquitousness of legionellae in water in the outdoor environment. Factors that influence survival in natural reservoirs include temperature, pH, available nutrients, and existing microbiota. Control in natural reservoirs is probably outside the scope of epidemiology and medicine and certainly outside the scope of building management practices.
Any natural or man-made system that provides suitable conditions for the growth and multiplication of Legionella is considered a potential amplifier. A few organisms (inoculum) arrive from the reservoir and find a niche where they can grow to higher levels (be amplified). Examples of man-made amplifiers include cooling towers and evaporative condensers,,, humidifiers, potable water heaters and holding tanks, and pipes containing stagnant warm water.,, Shower heads,,33 faucet aerators, and whirlpool baths may serve as amplifiers as well as disseminators.
In short, any water or water system with conditions favorable for Legionella growth is a potential amplifier. Since Legionella can multiply in both protozoa and alveolar macrophages (specialized infection fighting cells in the lung), some investigators believe that protozoa are the natural host of legionellae in the environment and that humans are accidental secondary hosts. Theoretically, the association of Legionella with protozoa in the environment may enable them to overwhelm alveolar macrophages in the human lung.
Some legionellae survive the routine water treatment used to produce potable water., Legionellae are carried in the treated drinking water to buildings where they enter and colonize the plumbing fixtures, especially of the domestic hot water system. Cooling towers and other wet-type-heat rejection systems also can become colonized if potable water containing legionellae (at very low numbers) is used as the source for their make-up water. This is probably the most frequent way equipment becomes contaminated even through some equipment, such as cooling towers, are excellent air scrubbers. Aerosolized legionellae contained in droplets may possibly be removed from the air and find a niche in open recirculating systems.
Most methods practiced for preventing the transmission of legionellae are at the man-made amplifier. If legionellae are prevented from amplifying in or on a device, the probability of having legionellae in sufficient concentrations to be an infectious dose for a susceptible host is greatly reduced. The following management strategies for amplifiers are directed at minimizing colonization, amplification within the equipment, or both:
1. Avoid piping that is capped and has no flow (dead legs). If such piping exists after renovation, it should be removed from both domestic and cooling water systems.
2. Control domestic water temperature to avoid temperature ranges where legionellae grow and to keep domestic cold water below 25° C (77° F) and hot water above 55° C (131° F). Measures to prevent scalding are essential.
3. Apply biocides in accordance with label dosages to control growth of other bacteria, algae, and protozoa that may contribute to nutritional needs of legionellae.
4. Limit available niches for microbiata. Managing existing microbiota can partially be addressed by maintaining clean systems, thus achieving this goal. Removing or preventing sediment accumulation in cooling basins, decorative fountains, or hot water tanks is an example of a method to minimize microbial niches.
Engineering management of potable systems should include:
1. Temperature control (see above).
2. Plumbing enhancements that may include self-draining shower lines, anti-scald valves, pipe insulation, or other devices that control water temperature.
3. Chlorination of the potable system (see Section 7.0, Emergency Decontamination for Legionella).
Another way to decrease transmission of legionellae is to prevent aerosolization. If legionellae are not aerosolized, they will not be carried by the air to people. Common building-associated equipment that generate aerosols include cooling towers, evaporative condensers, shower heads, faucet aerators, whirlpool baths, nebulizers, and humidifiers.
Engineering management of HVAC equipment is directed at three methods in addition to the four listed above:
1. Design cooling tower and building air intake placement so air discharged from the cooling tower or evaporative condenser is not directly brought into the building air intake.
2. Maintain effective drift eliminators on cooling towers and evaporative condensers.
3. Ensure air filters for outside air are dry. Water droplets or humidity that condenses on filters provide an environment where microbes can grow and could be dispersed to the conditioned space. Air filters should be cleaned or replaced as directed by the manufacturer.
Airborne transmission is generally accepted as the primary means by which legionellae are transferred to humans. In order for this to occur, aerosols of legionellae must be generated, and the ambient conditions of the air such as temperature, moisture, and solar radiation must not be too harsh. Survival of legionellae appears best under humid conditions (greater than 65% relative humidity). Size of the droplets produced influence the amount of time the organisms survive. When the droplets evaporate, cells dry and cell viability decreases. The distances that droplets travel and the time droplets are in the air are factors that, while poorly understood, are expected to influence organism viability when they are inhaled.
Public health officials stress that the mere presence of legionellae either in water or on a fixture or device will not in itself cause people who are present in the area to develop disease. For disease to occur, several conditions must exist simultaneously:
1. The legionellae must have sufficient virulence factors to cause disease. A number of factors found in certain strains of legionellae have been associated with virulence.
2. The virulent legionellae must be present in sufficient number to cause an infection. To date the infectious dose for humans has not been determined, although it probably varies according to the susceptibility of the potential host.
3. The potential host must inhale air contaminated with particles containing legionellae that are less than 5 µm in size. Infection will occur only if the legionellae reach the deepest parts of the lungs.
4. The host’s defense system is overwhelmed by the infection.
Factors influencing the susceptibility of humans to legionellosis have been studied in both epidemic and sporadic cases. In Pontiac fever, no conspicuous factors affecting susceptibility were noted. For Legionnaires’ disease, case rates are higher in males and older age groups, people who smoke, have chronic illness, or are under medical treatment regimes that are immunosuppressive; that is, depress the body’s defense mechanisms against bacteria. Some patients in hospitals and nursing homes are examples of people who may be at higher risk of legionellosis.
To develop disease people must be exposed to the causative organism. Among those who are exposed, the body’s protective defense systems often, but not always prevent entry of the organisms. If the organisms gain entry to the body, the defense systems often kill them before they establish an infection. Protective defense systems include mechanisms in the respiratory and digestive systems and on the skin. In persons with weakened defenses due to smoking, underlying diseases, medications, genetically determined factors, and possibly advanced age, the infecting organisms have a greater chance to overcome the body’s defenses and establish the disease.
In humans, the deposition of airborne particles in the respiratory tract is governed primarily by particle size. Particles 10 µm or more in diameter will deposit in the nose and throat. Particles between 5 µm and 10 µm will deposit both in the upper and lower respiratory tract. Particles under 5 µm but larger than 2 µm will deposit in the lung principally in lower airways. Particles 0.5 µm to 2 µm in size will deposit mainly in the gas exchange area, which is the deepest part of the lung. Some infections, such as legionellosis, can occur only if particles contaminated with virulent legionellae reach the deepest parts of the lung’s respiratory tree.
The upper part of the respiratory tract has a ciliated mucosal lining. Mucus is excreted around the particles that are deposited. Movement of the cilia of the respiratory tract expel the particles continuously in a wave-like motion; however, these mechanisms can be slowed or stopped by smoking or other forms of air pollution, by certain immunosuppressive drugs, and by some forms of illness. These predisposing conditions, called risk factors, increase the chance of individuals becoming infected with legionellae.
Although infectious dose responses have been determined for some laboratory animals, the infectious dose for humans has not been determined. A reasonable assumption is that individuals who have more risk factors should become infected with lower doses than individuals who have few or no risk factors.
Because contamination of some potable waters with legionellae have been documented, concern has been expressed that legionellosis could be contracted by drinking contaminated water, especially if it should be aspirated into the lungs. On rare occasions Legionnaire’s disease may have occurred through aspiration in hospitalized patients with in-dwelling nasogastric tubes.,
5.0 Preventative Strategies and Monitoring
Good preventative maintenance is very important in the efficient operation of cooling towers and other evaporative equipment. Preventive maintenance includes having effective drift eliminators, periodically cleaning the system if appropriate, maintaining mechanical components in working order, and having a water treatment program including a biocide. Note that most water treatment programs are designed to minimize scale, corrosion, and biofouling and not to control legionellae.
An estimated 25% to 40% of the 200,000 cooling towers employed on HVAC applications in the U.S. are neither well maintained (broken or missing drift eliminators, unbalanced fans, sludge in basins, and biofilm attached to surfaces) nor treated with biocides. Untreated systems have been correlated with an outbreak of legionellosis. Such conditions are not conducive to efficient system operation (biofouling may reduce heat exchanger efficiency) or to the reducing the risk of legionellosis transmission.
There is not enough information available to adopt a position on what is a "safe" or "unsafe" number of legionellae or how long that number would be valid for an individual dynamic system. Some cases of legionellosis indicate that a low number of legionellae was sufficient to cause illness. In other analyses, higher numbers of legionellae have not been correlated with disease. Directing research efforts at both numbers and virulent characteristics necessary for disease is recommended.
Culturing for Legionella may be appropriate if carried out for a specific purpose, such as tracing the source of an infection, evaluating the potential amplifier or transmission sources at a facility, verifying the effectiveness of a water treatment protocol, verifying that decontamination procedures have been effective, or in health care facilities caring for patients with exceedingly high risk of developing Legionnaire’s disease, such as organ transplant recipients or other high risk people.
Routine culturing or samples from building water systems for Legionella has limitations. The presence of the organism is not directly equated to the risk of infection, and the organism has been found without being associated with cases of disease. A number of factors, other than the concentration of organisms in a sample, influence the risk of illness following exposure. Factors include strain virulence, host susceptibility, and how efficiently the organisms are aerosolized to the small particle size required to reach the deep portion of the human lung. Conversely, lack of detection of the organism from a sample does not mean that the potential amplifier sampled will never be colonized. Variations in culture procedures among different labs can lead to different results. Additionally, use of fluorescent antibody or gene probes can result in different values from culture techniques for the same samples so interpretation of the data is important.
Periodic or routine monitoring is never an effective program if done in the absence of a maintenance program. MORE EMPHASIS SHOULD BE PLACED ON CLEAN EQUIPMENT IN EXCELLENT REPAIR THAN ON PERIODIC TESTING WITH CONCURRENT SYSTEM NEGLECT. Refer to Section 4.2, Amplifiers, and Section 4.3, Aerosolization, for specific measures to manage amplifiers and aerosols.
6.0 Safety Precautions for Decontamination Procedures
Although poorly documented in the literature, it must be stressed that several workers who have either serviced or cleaned wet-type-heat rejection equipment and other aerosol producing devices have experienced either Legionnaires’ disease or Pontiac fever. But, in another study increased risk for cooling system maintenance workers was not observed.
Workers conducting cleaning operations that are expected to produce aerosols are advised to wear a half-face respirator mask equipped with a cartridge filter that has a HEPA filter or "Type H" high efficiency rating. Filters capable of filtering aerosols, mists, particulates, radionucleotides, and asbestos should also be capable of removing Legionella and provide protection. Wearing the respirator mask is particularly advisable during cleaning if high-pressure steam, water, or air is applied to remove deposits. During decontamination procedures, workers also should wear rubber gloves, goggles, and protective clothing to prevent injury to their skin, eyes, and lungs by the chemicals being used to decontaminate equipment.
Also, air intake ducts should be closed during cleaning if they are within 30 m (100 ft) of a cooling tower being decontaminated. If humidifiers, drain pans, cooling coils, wet duct work, nebulizers, showers, or other indoor equipment are determined to need decontamination procedures, measures to minimize and contain aerosols are recommended. Since a variety of equipment and physical situations are possible, workers doing the decontamination should apply the information discussed in Section 3.0, The Disease, and Section 4.0, Transmission Factors and Prevention, to the specific circumstances at the site to protect themselves and occupants of the building or adjacent buildings.
7.0 Emergency Decontamination for Legionella
The Cooling Tower Institute has formulated an emergency protocol for decontaminating wet-type-heat rejection systems using chlorine and dispersants. This protocol also was developed to respond to systems suspected of containing the organisms that caused an outbreak. ASHRAE’s position is that this procedure must not be used routinely, because it can be very corrosive and produce toxic fumes. This procedure has been adapted to include additional safety procedures and 10 mg/L free residual chlorine level for 24 hours.
For emergency decontamination of potable water systems increasing water temperatures above 60° C or continuous chlorination at 1 ppm to 2 ppm free chlorine at the tap for several hours may work. Long-term decontamination is not provided by this procedure.
Based on the scientific, medical, and engineering literature available to date, ASHRAE concludes the following:
1. Heating, ventilating, air-conditioning and refrigerating (HVAC&R) systems and their components as well as potable hot water services and bathing equipment can amplify and disseminate aerosols of a wide variety of airborne contaminants, including Legionella bacteria, and the agents causing legionellosis (Legionnaires’ disease and Pontiac fever).
2. Design and operation and maintenance procedures that prevent amplification and dissemination of Legionella should be formulated and implemented before systems are operated and continued rigidly thereafter. Although using these practices will not guarantee that a system or individual component will be free of legionellae, they should reduce the chance of the systems becoming heavily colonized with these bacteria.
3. Currently, the only reliable method of testing for the presence of legionellae in a system is by culturing for these organisms. No "non-legionellae" surrogate tests are available, and there is currently no correlation between total bacterial counts and legionellae concentrations. The results of single tests must be interpreted cautiously as the concentration of Legionella in a water system can increase substantially over a few days. Attention should be paid to sample handling and laboratory quality control so that the number reported reflects the number present at the time of sampling.
4. The efficacy of a specific biocide treatment in controlling legionellae can only be determined by testing specifically for the presence of legionellae in the field under actual working conditions. Laboratory trials must not be relied on exclusively as the sole proof of the efficacy of a biocide.
1Hoge, C.W. and R.F. Breiman. 1991. The epidemiology and control of Legionella infections: an update. Epidemiol rev. 13: 329-340.
2 Blackmon, J.A., R.W. Chandler, W.B.Cherly, A.C.England,III, J.C.Feeley, MD. Hicklin, R.M. McKinney and H.W. Wilkinson. 1981. Review Article: Legionellosis. Amer.J.Pathol.102:427-465.
3 Fallon, R.J., D.J.Goldberg, J.G.Wrench, S.T.Green, and J.A.N.Emslie. 1993. Pontiac fever in children. In J.M.Barbaree, R.F.Breiman, and A.P.Dufour (ed.), Legionella: Current Status and Emerging Perspectives, pp 50-51. American Society for Microbiology, Washington, D.C.
4 Centers for disease Control and Prevention. 1994. Procedures for the recovery of Legionella from the environment.
5 Fliermans, C.B., W.B.Cherry, L.H.Orrison, S.J.Smith, D.L.Tison, and D.H.Pope. 1981. Ecological distribution of Legionella pneumophila. Appl. Environ.Microbiol.41:9-16.
6 Fliermans,C.B. W.B.Cherry, L.H.Orrison, L.H.Thacker. 1979. Isolation of Legionella pneumophila from nonepidemic-related aquatic habitats. Appl.Environ.Microbiol. 37:1239-2342.
7 Stout,J.E., V.L.Yu, and M.G.Best. 1985. Ecology of Legionella pneumophila within water distribution systems. Appl.Environ.Microbiol 49:21-228.
8 Pope,D.H., R.J.Soracco, H.K.Gill and C.B.Fliermans. 1982. Growth of Legionella pneumophila in two membered cultures with green algae and cyanobacteria. Curr.Microbiol 7:319-322.
9 Anand,C.M., A.R.Skinner, A.Malic and J.B.Kurta. 1983. Interaction of L.pneumophila and a free living amoeba (Acanthamoeba palestinensis). J.of Hygiene 91:167-178.
10 Barbaree,J.M., B.S. Fields, J.C.Feeley, G.W.Gorman, and W.T.Martin. 1986. Isolation of protozoa from water associated with a Legionellosis outbreak and demonstration of intracellular multiplication of Legionella. Appl. Environ. Microbiol 51:422-424.
11 Joly, J.R., R.M.McKinney, J.O.Tobin, W.F.Bibb, I.D. Watkins and D.Ramsay. 1986. Development of a standardized subgrouping scheme for Legionella pneumophila serogroup 1 using monoclonal antibodies. J.Clin.Microbiol. 23:768-771.
12 Selander, R.K., R.M.McKinney, T.S.Whittam, W.F.Bibb, D.J.Brenner, F.S.Nolte, and P.E.Pattison. 1985. Genetic structure of a population of Legionella pneumophila. J.Bacteriol. 163:1021-1037.
13.Mamolen, M., R.F.Breiman, J.M.M.Barbaree, R.A.Gunn, K.M.Stone, J.S.Spika, D.T.Dennis, S.H.Mao, and R.L.Vogt. 1993. Use of multiple molecular subtyping techniques to investigate a Legionnaires’ disease outbreak due to identical strains at two tourist lodges. J.Clin.Microbiol. 31:2584-2588.
14 Streulens, J.J., N.Maes, A.Deplano, N.Bornstein, and F.Grimont. 1993. Comparison of methods for subtyping Legionella pneumophila. In J.M.Barbaree, R.F.Breiman and A.P.Dufour (ed), Current Status and Emerging Perspectives, pp183-186. American Society for Microbiology, Washington, D.C.
15 Gomez-Lus, P., B.S.Fields, R.F.Benson, W.T.Martin, S.P.O’Connor and C.M.Black. 1993. Comparison of arbitrarily primed polymerase chain reaction, ribotyping and monoclonal antibody analysis for subtyping Legionella pneumophila serogroup 1. J.Clin.Microbiol 31:1940-1942.
16 Schoonmaker, D., T.Heimberger and G.Birkhead. 1992. Comparison of ribotyping and restriction enzyme analysis using pulsed-field-gel electrophoresis for distinguishing Legionella pneumophila isolates obtained during a nosocomial outbreak. J.Clin.Microbiol. 31:1491-1498.
17 Barbaree, J.M. 1993. Selecting a subtyping technique for use in investigations of legionellosis epidemics. In J.M.Barbaree, R.F.Breiman, and A.P.Dufour (ed). Legionella: Current Status and Emerging Perspectives pp.169-172. American Society for Microbiology, Washington, D.C.
18 Yee,R.B. and R.M.Wadowsky. 1982. Multiplication of Legionella pneumophila in unsterlized tap water. Appl.Env.Microbiol. 43:1330-1334.
19 Fraser,D.W., T.R.Tsai, O.Orenstein, W.E.Parkin, J.J.Beecham, R.G.Sharrar, J.Harris, G.F.Mallison, S.M.Martin, J.E.McDade, C.C.Sheppard, and P.S.Brachman. 1977. Field investigation team: Legionnaires’ disease: description of an epidemic of pneumonia. N.Engl.J.Med. 297:1189-1197.
20 McDade,J.E., D.J. Brenner, F.M.Bozeman. 1979. Legionnaires’ disease bacterium isolated in 1947. Ann.Intern.Med. 90:659-661.
21 Osterholm,M.T., T.D.Y. Chin, D.O.Osborne, H.B.Dull, A.G.Dean, D.W.Fraser, P.S.Hayes, W.N.Hall. 1983. A 1957 outbreak of Legionnaires’ disease associated with a meat packing plant. Am.J.Epidem. 117:63-67.
22Horwitz, M.A., B.J.Marston, C.V.Broome and R.F.Breiman. 1993. Prospects for Vaccine Development. In J.M.Barbaree, R.F.Breiman and A.P.Dufour (eds). Legionella: current Status and emerging Perspectives. American Society for Microbiology, Washington, D.C.
23 Bartlett, C.L.R., A.D.Macrae, and J.T.MacFarlane. 1986. Legionella Infections. Edward Arnold, London.
24 Cordes,L.G., W.D.Goldman, J.S.Marr, S.M.Friedman, J.D.Band, E.O.Rothschild, H.Kravetz, J.C.Feeley, and D.W.Fraser. 1980. Field investigation team: Legionnaires’ disease in New York City. August-September 1978. Bull NY Acad.Med. 56:467-482.
25 Band, J.D. M.LaVenture, J.P.Davis, G.F.Mallison, W.L.Schell, P.Skaliy, P.S.Hayes, H.Weiss, D.J.Greenberg, and D.W.Fraser. 1981. Epidemic Legionnaires’ disease: airborne transmission down a chimney. J.A.M.A. 254:2404-2407.
26 Broome, C.V., S.A.T.Goings, S.B.Thacker, R.L.Vogt, H.N.Beaty and D.W.Fraser. 1979. Field Investigation team: the Vermont epidemic of Legionnaires’ disease. Ann.Intern.Med. 90:573-577.
27 Cordes,L.G., D.W.Fraser, P.Skaliy, C.A.Perlino, W.R.Elsea, G.F.Mallison, P.S.Hayes. 1980. Legionnaires’ disease outbreak at an Atlanta, Georgia country club: evidence for spread from an evaporative condenser. Am.J.Epidemiol 111:425-431.
28 Dondero,R.J.,Jr., R.C.Rendtorff, G.F.Mallison, R.M.Weeks, J.S.Levy, E.S.Wong, W.Schaffner. 1980. An outbreak of Legionnaires’ disease associated with a contaminated air conditioning cooling tower. N.Engl.J.Med. 302:365-370.
29 Garbe,P.L., B.J.Davis, J.S.Weisfeld, L.Markowitz, P.Miner, F.Garrity, J.M.Barbaree and A.L.Reingold. 1985. Nosocomial Legionnaires’ disease-epidemiologic demonstration of cooling towers as a source. J.Am.Med.Assoc. 254:521-524.
30 Best,M., V.L.Yu, J.Stout, A.Goetz, R.R.Muder and F.Taylor. 1983. Legionellae in the hospital water supply epidemiological link with disease and evaluation of a method of control of nosocomial Legionnaires’ disease and Pittsburgh pneumonia. Lancet (Aug 6)307-310.
31 Muder,R.R., V.L.Yu, A.H.Woo. 1986. Mode of transmission of Legionella pneumophila Arch.Intern.Med. 146:1607-11.
32 Witherell, L.E., L.A.Orciari, K.C.Spitalny, R.A.Pelletier, K.A.Stone, and R.L.Voght. 1984. Disinfection of Legionella pneumophila contaminated whirlpool spas. In C.Thronsberry, A.Balows, J.C.Feeley and W.Jakubowski (ed). Legionella: Proceedings of the 2nd International Symposium. American Society for Microbiology. Washington, D.C.
33 Patterson, W.J., D.V.Seal, E.Curran, T.M.Sinclair, and J.C.McLuckie. 1994. Fatal nosocomial Legionnaires’ disease: relevance of contamination of hospital water supply by temperature-dependent buoyancy-driven flow from spur pipes. Epidemiol.Infect. 112:513-525.
34 Brundrett, G.W. 1992. Legionella and Building Services. Butterworth-Heinmann, Ltd. Oxford, United Kingdom.
35 Gorman, G.W., V.L.Yu, A.Brown, J.A.Hall, L.K.Corcoran, W.T.Martin, W.F.Bibb, G.K.Morris, M.H.Magnussen and D.W.Fraser. 1980. Isolation of Pittsburgh pneumonia agent from nebulizers used in respiratory therapy. Ann.Itnern.Med. 93(4):572-573.
36 Fenstersheib, M.D., M.Miller, C.Diggins, S.Liska, L.Detwiler, S.B.Werner, D.Lindquist, W.L.Thacker, R.F.Benson. 1990. Outbreak of Pontiac fever due to Legionella anisa. Lancet 336:35-37.
37 Hady, W.G., R.C.Mullen, C.S.Mintz, B.G.Shelton, R.S.Hopkins and G.L.Daikos. 1993. Outbreaks of Legionnaires’ disease linked to a decorative fountain. Am.J.Epidemiol. 138:555-562.
38 Mahoney, F.J., C.W.Hoge, T.A.Farley, J.M.Barbaree, R.F.Breiman, R.F.Benson, and L.M.McFarland. 1992. Communitywide outbreak of Legionnaires’ disease associated with a grocery store mist machine. J.Infect.Diseas. 165:736-739.
39 Glick, T.H., M.G.Gregg, B.Berman, G.Mallison, W.W.Rhodes and I.Kassanoff. 1978. Pontiac fever: an epidemic of unknown etiology in a health department. 1. Clinical and epidemiologic aspects. Am.J.Epidemiol. 107:149-160.
40 Kaufmann, A.K., J.E.McDade, C.M.Patton, J.V.Bennett, P.Skaliy, J.C.Feeley, D.C.Anderson, M.E.Potter, V.F.Newhouse, M.B.Gregg and P.S.Brachman. 1981. Pontiac fever: demonstration of its mode of transmission. Am.J.Epidemiol. 114:337-374.
41 Friedman, S., K.Spitalmy, J.Barbaree, Y.Faur, and R.McKinney. 1987. Pontiac fever outbreak associated with a cooling tower. Am.J.Public Health 77:568-571.
42 Fields, B.S., J.M.Barbaree, G.N.Sanden, and W.E.Morrill. 1990. Virulence of Legionella anisa strain associated with Pontiac fever: an evaluation using protozoan, cell culture and guinea pig models. Infect.Immun. 58:3139-3142.
43Herwaldt, L.A., G.W.Gorman, T.McGrath, S.Toma, B.Brake, A.W.Hightower, J.Jones, A.L.Reingold, P.A.Boxer, P.Tang, C.W.Moss, H.W.Wilkinson, D.J.Brenner, A.G.Steigerwalt and C.V.Broome. 1984. A new Legionella species, Legionella feleii species nova, causes Pontiac fever in an automobile plant. Am.Intern.Med. 100:333-338.
44Barbaree, J.M. 1991. Controlling Legionella in cooling towers. ASHRAE J.33:(6)38-42.
45Dutka, B.J., K.Walsh, P.Ewan, A.El-Shaarawi and R.S.Tobin. 1983. Environmental distribution patterns of Legionella in central Canada and longevity studies. National Water Research Institute manuscript no. 69-AMD-5-83-BID Canada Centre for Inland Waters, Burlington, Ontario, Canada.
46Tison, D.L., and Seidler, R.J. 1983. Legionella in aquatic habitats in the Mount Saint Helen’s blast zone. Curr.Microbiol. 9:345-348.
47 Shelton, B., D.Flanders, G.Morris. 1994. Legionnaires’ disease outbreaks and cooling towers with amplified Legionella concentrations. Current Microbiol. 28:359-363.
48 Witherell, L.E., L.F.Novick, K.M.Stone, R.W.Duncan, L.A.Orciari, S.J.Kappel, D.A.Jilson. 1986. Legionella in cooling towers. J.Env.Health. 49(3):134-139.
49 Broadbent, C.R. 1997. Water Treatment. Mechanical Engineering Services Application Manual. Australian Institute of Refrigeration, Air-Conditioning and Heating.
50 Tyndall, R.L., E.S.Lehman, E.K.Bowman, D.K.Milton, J.M.Barbaree. 1995. Home humidifiers as a potential source of exposure to microbial pathogens, endotoxins, and allergens. Indoor Air 5:171-178.
51 Patterson, W.J., D.V.Seal, E.Curran, T.M.Sinclair, and J.C.McLuckie. 1994. Fatal nosocomial Legionnaires’ disease: relevance of contamination of hospital water supply by temperature-dependent buoyancy-driven flow from spur pipes. Epidemiol.Infect. 112:513-525.
52Freije, M.R. 1996. Legionella Control in Health Care Facilities. A Guide for Minimizing Risk. HC Information Resources, Inc. Indianapolis, IN.
53 Chartered Institution of Building Services Engineers. 1991. Minimizing the Risk of Legionnaires’ disease. Technical Memoranda 13. London.
54 Breiman, R.F., B.S.Fields, G.N.Sanden, L.Volmer, A.Meir, J.S.Spika. 1990. Association of shower use with Legionnaires’ disease: possible role of amoebae. JAMA 263 (21):2924-2926.
55 Jernigan, D.B., J.Hofmann, M.S.Cetron, C.A.Genese, J.P.Nuorti, B.S.Fields, R.F.Benson, R.J.Carter, P.H.Edelstein, J.C.Gurrero, S.M.Paul, H.B.Lipman, R.F.Breiman. 1996. Outbreak of Legionnaires’ disease among cruise ship passengers exposed to a contaminated whirlpool spa. Lancet. 347:494-500.
56 Shands, J., J.Ho, R.Meyer, G.Gorman, G.Mallison, S.Finegold and D.Fraser. 1985. Potable water as a source of Legionnaires’ disease. JAMA. 253:1412-1416.
57.States, S.J., L.F.Conley, J.M.Kuchta, B.M.Oleck, M.J.Lipovich, R.S.Wolford, R.M.Wadowsky, A.M.McNamara, J.L.Sykora, G.Keleti, R.B.Yee. 1987. Survival and multiplication of Legionella pneumophila in municipal drinking water systems. Appl.Env.Microbiol. 53:979-986.
58 Berendt, R.F. 1980. Survival of Legionella pneumophila in aerosols: effect of relative humidity. J.Infect.dis.141:689.
59 Broome, C.V. 1983. Current issues in epidemiology of Legionellosis. In C.Thornsberry, A.Balows, J.C.Feeley and W.Jakubowski (eds) Legionella Proceedings of the 2nd International Symposium. American Society of Microbiology. Washington, D.C.
60 Brendt, R.F., H.W.Young, R.G.Allen and G.L.Knutsen. 1980. Dose response of guinea pigs experimentally infected with aerosols of Legionella pneumophila. J.Infect.Dis. 141:689.
61 Yu, V.L. 1993. Could Aspiration be the major mode of transmission for Legionella? Am.J.Med.95:13-15.
62 Blatt, S.P., M.D.Parkinson, E.Pace, P.Hoffman, D.Dolan, P.Lauderdale, R.Zajac, G.Melcher. 1993. Nosocomial Legionnaires’ Disease: Aspiration as a Primary Mode of Disease Acquisition. Am.J.Med. 95:(7)16.
63 Venezia, R.A., M.D.Agresta, E.M.Hanley, K.Urquhart, D.Schoonmaker. 1994. Nosocomial Legionellosis Associated with Aspiration of Nasogastric Feedings Diluted in Tap Water. Infec.Control.Hosp.Epidermiol. 15:529-533.
64 American Society of Heating, Refrigerating and Air-Conditioning Engineers. 1996. Systems and Equipment Handbook 33b.10-13. Atlanta, GA.
65 Mitchell,P., A.Chereshsky, A.J.Haskell, M.A.Briesman. 1991. Legionellosis in New Zealand: first recorded outbreak. New Zealand Med.J. 104:915-916.
66 OSHA. Section 11 Chapter 7 Legionnaires’ Disease.
67 Fraser, D.W., D.C.Beubner, D.L.Hill, D.K.Gilliam. 1979. Nonpneumonic, short-incubation-period Legionellosis (Pontiac fever) in men who cleaned a steam turbine condenser. Science 205:690-691.
68 Goldman, W.D. and J.S.Marr. 1980. Are air-conditioning maintenance personnel at increased risk of legionellosis? Appl.Env.Microbiol. 40:114-116.
69 Cooling Tower Institute. 1980. Suggested protocol for emergency cleaning of cooling towers and related equipment suspected of infection by Legionnaires’ disease bacteria (pneumophila). Houston, TX.
70 Tablan, O.C., L.J.Anderson, N.H.Arden, R.F.Breiman, J.C.Butler, M.M.McNeil and the Hospital Infection Control Practices Advisory Committee. 1994. Guideline for prevention of nosocomial pneumonia. Part 1. Issues on prevention of nosocomial pneumonia. Am.M.Infect.Control
Revision Date: August 3, 1998
|
©1998 ASHRAE. All Rights reserved. |